Winkelwagen
U heeft geen artikelen in uw winkelwagen
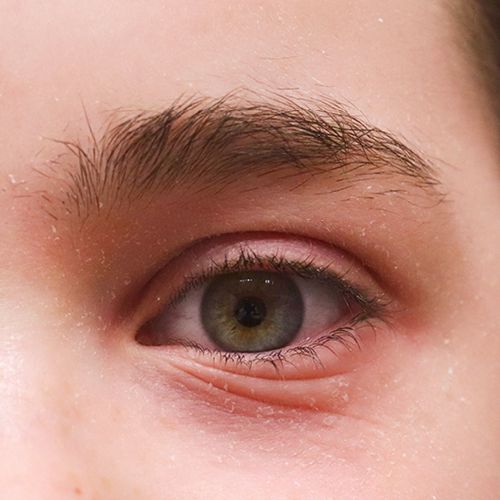
Eczeem heeft een grote invloed op het welzijn van degenen die eraan lijden - vooral wanneer opvallende plekken zoals het gezicht zijn getroffen. DERMASENCE heeft de Vitop forte verzorgingslijn ontwikkeld met het unieke Vitop forte-complex van wede, aloë vera en groene thee wat een kalmerende werking heeft bij de droge, geïrriteerde huid die gevoelig is voor roodheid en jeuk.
Een effectieve huidverzorgingsroutine voor eczeem in het gezicht begint met een milde reiniging. Onze DERMASENCE Vitop forte Cleansing foam behoudt de vochtbalans en de pH-waarde van de huid. De zeepvrije formule is bijzonder zacht voor de geïrriteerde huid.
Een kleine skinhack: onze DERMASENCE Vitop forte Eye care kun je ook gebruiken voor de verzorging van eczeemgevoelige lippen!
Doordat de huidbarriére bij atopisch eczeem minder goed werkt en daardoor de huid vaak stuk is ontstaan er gemakkelijker infecties.

Zowel de plaats waar atopisch eczeem op het lichaam voorkomt, als de verschijningsvorm zijn afhankelijk van de leeftijd van de persoon en het stadium waarin het eczeem verkeert.
– Acuut eczeem: We zien roodheid, zwelling, vochtblaasjes, natten en krabeffecten. Daarna drogen de blaasjes in tot korstjes, gaat de huid schilferen en neemt de roodheid af.
– Chronisch eczeem: De roodheid neemt af, de schilfering neemt toe en de huid is wat dikker. De huidlijnen worden grover dan normaal, dit heet “lichenificatie”. In de stugge en/of droge huid kunnen (pijnlijke) kloven ontstaan.
Een typische en vervelende eigenschap van eczeem is dat het altijd in meer of mindere mate jeukt. Hierdoor krabt de patiënt aan de huid . Door het wrijven en krabben wordt het eczeem echter juist verergerd en in stand gehouden. Ook is de huid meestal droog, een droge huid geeft weer aanleiding tot jeuk.
– Baby’s: 4 weken tot 1 jaar, acuut eczeem, d.w.z nattend, blaasjes, korstjes, met felle roodheid en veel jeuk, in het gelaat (dauwworm) , romp en ledematen
– Kinderleeftijd, 1 jr tot 14 jr, gelaat, elleboogplooien en knieholtes, polsen en enkels: roodheid, bultjes, blaasjes, ook droog, kloofjes, verdikking en vergroving huid
– Volwassen leeftijd, vanaf 14 jr: chronisch terugkerend eczeem met periodes van rust en verergeringen . Vooral droog, schilferend met vergroving huid.
1. Prurigo van Besnier: heftige jeukende bulten op de romp en strekzijden ledematen